
托马斯·邓肯博士
语言
WEB资源
研究项目和附属机构
研究兴趣
F-type ATP合酶s; bioenergetics of pathogenic bacteria; enzymology; structural biology, 膜蛋白功能.
教育利益
Bioenergetics; mitochondria; lipid and cholesterol metabolism
协会/会员
教育
研究抽象
我的研究重点是f型ATP合酶的结构和功能, an energy-transducing enzyme complex that is critical to the energy metabolism of most living things. My current emphasis is on its critical roles in bacterial metabolism, virulence and pathogenesis. f型ATP合酶是在细菌的内细胞膜上组装的, the inner mitochondrial membrane of eukaryotes and on the thylakoid membrane of chloroplasts (see example for the ‘simple’ bacterial enzyme, 无花果. 1). 在氧化磷酸化和光磷酸化的最后一步, 它利用跨膜的能量, 质子的电化学梯度(质子动力), or 及)来驱动大多数细胞ATP的合成. However, the enzyme is intrinsically reversible and, if the driving force is lost for respiration (e.g.,缺乏2) or photosynthesis (darkness), the enzyme can shift to hydrolyzing cellular ATP, driving proton (H+)以相反的方向在膜上运输并产生 及. The enzyme is critical for the viability and/or virulence of diverse pathogenic bacteria, some of which rely on it for ATP synthesis while others can only use it as an ATPase-driven H+ 泵. Despite the general structural/functional conservation between bacterial and eukaryotic forms of the enzyme, the potential to target the enzyme to attack pathogenic bacteria has been confirmed by the development of Bedaquiline, 被发现是针对F分枝杆菌的OF1 目前被批准为三种药物的一部分, 治疗耐多药结核病的一线方案. Bedaquiline is the first in a new diarylquinoline class of antibiotics that is highly selective for mycobacterial FOF1.
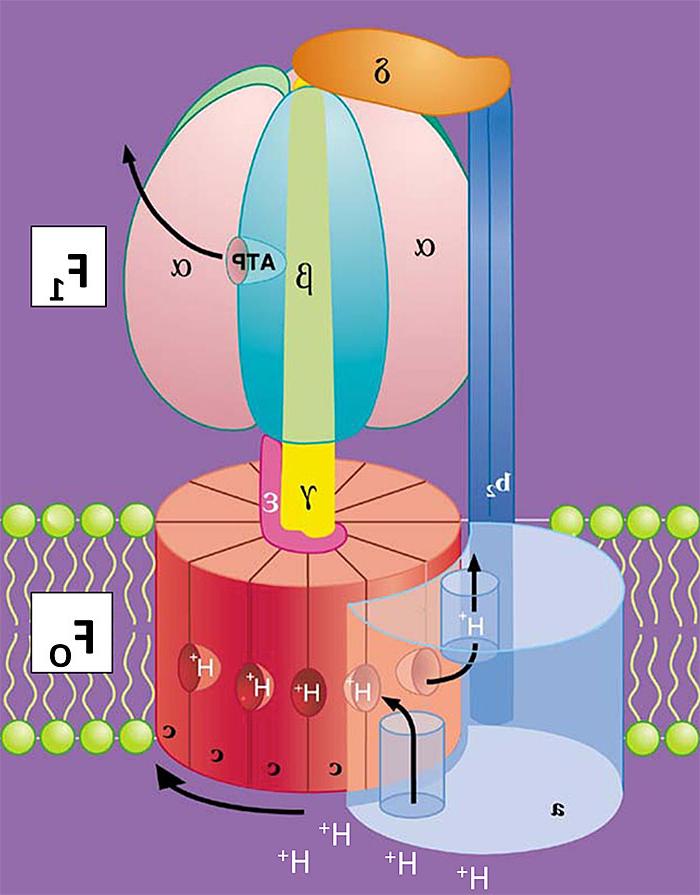
无花果. 1. E的漫画. 杆菌FOF1 体系结构. 箭头下方的c环表示方向转子复合体(c10在ATP合成过程中,γε)在H+ 运输.
BACKGROUND
The ATP合酶 consists of two sub-complexes with distinct but coupled functions. 酶的基本结构以卡通形式显示在 无花果. 1 在大多数细菌中发现的最小亚基组成. FO 络合物催化H+ 转运并具有跨膜双层的亚基. F1 is a peripheral complex that extends into the aqueous phase and contains three catalytic nucleotide-binding sites for synthesis and hydrolysis of ATP. FO 和F1 are coupled through two stalk-like connections of subunits: a central rotor shaft and a peripheral stator. My earlier studies with Richard Cross provided key initial evidence that energy coupling by the ATP合酶 involves
- 中心轴在F内的旋转1 to coordinate actions of the three cooperative, alternating catalytic nucleotide sites, and
- F内亚基的旋转O 在能量驱动的质子传输过程中.
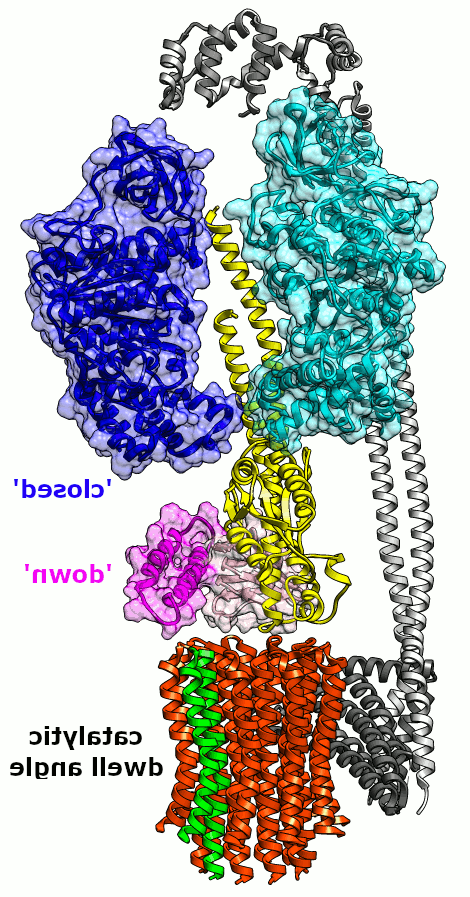
无花果. 2. 最小的 E. 杆菌 FOF1 动画. Likely conformational transitions of one ß subunit 和 εCTD relative to one small (~36°) rotary sub-step. 一个c亚基是绿色的,用来跟踪旋转 c-环沿转子轴转动 γ(黄色)和ε (NTD/CTD,粉色/品红). Only 1 other ß (cyan) is shown, 和 intervening alpha subunits are not shown.
实验系统 & 当前的项目
我们的研究长期使用来自E. 杆菌, which provides a simple yet powerful model for structure/function studies of this rotary motor enzyme. E. 杆菌 system allows straightforward genetic screening and engineering of the ATP合酶, 以及F的大规模提纯OF1 分离的可溶性F1 用于生物化学和结构研究. 我们继续发展更详细的知识 E. 杆菌 FOF1 to underst和 inhibitory mechanism of the εCTD and how to best target it to disrupt bacterial bioenergetics. 例如,我们正在与一位单分子显微镜专家合作, Dr. 迈克尔·罗宋汤 (耶拿大学, Germany) is using fluorescence resonance energy transfer (FRET) to study the dynamics of ε’s conformational changes in single EcFOF1脂质体.
我们对F的了解要少得多OF1 from other pathogenic bacterial species, and we have begun to study the function and impact of FOF1 在两种不同的病原体中:
- 铜绿假单胞菌. 这革兰氏阴性, opportunistic pathogen is a major contributor to persistent lung infections and mortality in cystic fibrosis patients. It is a substantial hazard for immune-compromised persons and is prevalent in hospital-acquired infections. 耐多药菌株不断出现, in 2017, 世界卫生组织列出的碳青霉烯耐药性 P. 绿脓杆菌 作为迫切需要开发新抗生素的三种病原体之一. P. 绿脓杆菌 metabolism is strongly dependent on respiration 和 ATP合酶 是必不可少的 its viability. 我们目前正在与 克里斯托弗。野村 在邻近的SUNY-ESF和 Guirong王 (推荐最近最火的赌博软件 department of Surgery)研究(i) F的εCTD如何OF1 影响酶的功能 P. 铜绿菌体外培养 and (ii) whether disruption of the εCTD impacts the virulence or pathogenesis of P. 绿脓杆菌 在培养的巨噬细胞和小鼠肺部感染模型中.
- 链球菌引起的肺炎. This gram-positive pathogen is the major cause of bacterial pneumonia but can also lead to severe invasive pneumococcal diseases such as sepsis and meningitis. This aerotolerant anaerobe lacks a complete respiratory chain, so it cannot use FOF1 重要的ATP合成. 然而,FOF1 是必不可少的 S. 肺炎的 活力和耐酸性,作为atp酶驱动的H+ 泵 to generate 及 across the cell membrane and to maintain cellular pH homeostasis. 研究小组最近对小鼠败血症进行了研究 马可Oggioni (University of Leicester, UK), suggested a significant virulence role of pneumococcal FOF1: S的亚克隆. pneumoniae that emerged to establish septicemia most often contained a nascent missense mutation in a gene for one of the subunits of FOF1 (2014, Gerlini et al ..中国生物医学工程学报(英文版). 相反,其中一个这样的毒性克隆在基因中发生了帧移突变 atpC ε基因打乱了ε ctd的序列, 这个肺炎球菌克隆在脾脏中显示出更高的存活率. 他们转移了 atpC 突变成naïve, 非包封肺炎球菌菌株, and we are working with it to determine how this disruption of the εCTD affects FOF1 在肺炎球菌膜中的功能.
选择引用
T.M. 邓肯 (2019). 涡轮酶的结构瞄准了肺结核. Proc. 国家的. 阿德莱德大学. Sci. 美国 116, 3956-58. http://doi.org/10.1073/pnas.1900798116
M. 索R. Ishmukhametov J.C. bouw,. 昨天,C. Suarna N.J. 史密斯,米. 克里斯蒂,R. 装料工T.M. 邓肯, A.G. 斯图尔特(2019). 低温电镜显示不同的构象 E. 杆菌 ATP合酶对ATP的影响. eLife 8, e43864. http://doi.org/10.7554/eLife.43864
H. Sielaff T.M. 邓肯, M. 罗宋汤(2018). 调节亚基ε in 大肠杆菌 FOF1atp合酶. Biochim. Biophys. Acta Bioenerg. 1859, 775-788. http://doi.org/10.1016/j.bbabio.2018.06.013
N.B. 沙,T.M. 邓肯 (2015). 好氧生长 大肠杆菌 is reduced and ATP synthesis is selectively inhibited when five C-terminal residues are deleted from the ε subunit of ATP合酶, J. 医学杂志. 化学. 290, 21032-41. http://doi.org/10.1074/jbc.M115.665059
M. 罗宋汤,T.M. 邓肯 (2013). 射灯电机和控制单FOF1atp合酶. 物化学. Soc. 反式. 41, 1219-26. http://doi.org/10.1042/BST20130101
N.B. 沙,M.L. 哈钦,B.K. 哈雾,T.M. 邓肯 (2013). F1腺苷三磷酸酶的 大肠杆菌: ATP水解后形成ε-抑制态, 与adp抑制状态不同, 并对催化位点配体做出动态反应. J. 医学杂志. 化学. 288, 9383-95. http://doi.org/10.1074/jbc.M113.451583
G. Cingolani T.M. 邓肯 (2011). ATP合酶催化复合物的结构(F1) 大肠杆菌 自我抑制的构象. Nat. Struc. 摩尔. 医学杂志. 18, 701-7. http://doi.org/10.1038/nsmb.2058
T.M. 邓肯 (2004). ATP合酶:旋转马达的部件和特性,见: 酶,卷. 二十三:能量耦合与分子马达,卷. 23, (D.D. 哈克尼F. Tamanoi, Eds.爱思唯尔学术出版社,纽约,第2页. 203-275.
M.L. 哈钦T.M. 邓肯, H. Ngai, R.L. 十字架(2001). 能量驱动亚基在亚基之间的界面旋转 a 和 c F的低聚物O 部门的 大肠杆菌 ATP合酶. Proc. 国家的. 阿德莱德大学. Sci.. 美国 98, 8519-24. http://doi.org/10.1073/pnas.151236798
T.M. 邓肯, V.V. Bulygin Y. 周,M.L. 哈钦,R.L. 十字架(1995). 催化过程中亚基的旋转 大肠杆菌 F1腺苷三磷酸酶. Proc. 国家的. 阿德莱德大学. Sci. 美国 92, 10964-68. http://doi.org/10.1073/pnas.92.24.10964
